Abstract
Bitopertin is an orally available selective inhibitor of glycine transporter 1 (GlyT1) that is being developed for the treatment of erythropoietic protoporphyria (EPP). Previously, bitopertin has been evaluated in the treatment of neuropsychiatric diseases in a series of studies that enrolled approximately 4,000 subjects. In these studies, bitopertin was well tolerated but ultimately this program was unsuccessful because of lack of efficacy.
Patients with erythropoietic protoporphyria (EPP) have mutations in the FECH gene that encodes ferrochelatase, the final enzyme in heme biosynthesis, resulting in the accumulation of the heme synthesis intermediate protoporphyrin IX (PPIX) in red cells. PPIX that is released from RBCs then accumulates in tissues. Cutaneous accumulation of PPIX causes painful photosensitivity that has major negative impacts on the quality of life of affected individuals. Accumulation of PPIX in the liver and biliary tract causes cholestasis that can lead to liver fibrosis and severe hepatopathy. Clinically evident hepatobiliary disease occurs in up to 20% of EPP patients with 5% progressing to cholestasis potentially leading to liver failure.
Glycine is the one of the two substrates for the initial step of heme biosynthesis. Glycine uptake via GlyT1 is required in erythroid precursors to meet the high demand of heme synthesis in these cells (Garcia-Santos et al., Haematologica 2017; Volume 102:1314). The effect of bitopertin on glycine uptake in erythroid cells was evaluated using an ex vivo glycine uptake assay. Bitopertin treatment reduced glycine uptake in mice with a maximum inhibition of 55% and an EC50 of 4 nM. In Wistar rats, in which bitopertin has a more favorable pharmacokinetic profile that is similar to humans, bitopertin treatment reduced glycine uptake up to 67% with an EC50 of 2 nM. By substantially reducing glycine uptake into erythroid precursors, bitopertin has the potential to reduce the primary source of pathogenic PPIX accumulation that leads to phototoxicity and hepatobiliary disease in patients with EPP.
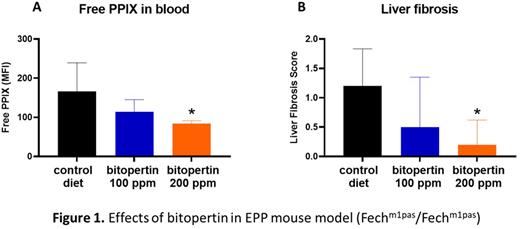
The potential of bitopertin to treat EPP was evaluated in male Fechm1Pas EPP mice. The recessive Fechm1Pas allele is an ethylnitrosourea (ENU)-induced missense mutation that retains approximately 5% residual ferrochelatase activity. Fechm1Pas/Fechm1Pas homozygous mice develop protoporphyria characterized by elevated PPIX levels in RBC and liver, cutaneous phototoxicity (Wang et al., Sci. Adv. 2019; 5 : eaaw6127) and liver fibrosis (Lyoumi et al, Gastroenterology 2011;141:1509). Gain-of function mutations in first gene of the heme synthesis pathway, 5'-Aminolevulinate Synthase 2 (ALAS2), also results in elevated PPIX levels and the development of protoporphyria in XLPP patients. Our previous studies of bitopertin in female Fechm1Pas/Fechm1Pas homozygous EPP mice and in male Alas2Q548X/Y XLPP mice demonstrated reduced PPIX accumulation in blood. To extend these observations, a cohort of male Fechm1Pas/Fechm1Pas mice was fed bitopertin (0, 100 or 200 ppm bitopertin) for 8 weeks, beginning at 8 weeks of age. At the study end, RBC PPIX levels had decreased in Fechm1Pas mice receiving bitopertin with a mean reduction of 32% and 50%, respectively at 100ppm and 200ppm, compared to the control group (Fig.1A). Histopathological assessment of liver sections showed evidence of cholestasis in the control group with bile accumulation, ductular reaction and bridging liver fibrosis that was evident with trichrome or reticulin staining. A score of 0-4 on the status of liver fibrosis was assigned to each liver section (0=no fibrosis, 1=portal fibrosis, 2= portal and periportal fibrosis, 3=bridging fibrosis, and 4=cirrhosis). Addition of bitopertin to the diet reduced the incidence of ductular reaction and the severity of liver fibrosis (Figure 1B).
In conclusion, we demonstrate that by inhibiting glycine uptake into erythroid precursors, bitopertin can reduce PPIX accumulation in blood and reduce histopathological evidence of cholestasis and liver fibrosis in the Fechm1Pas EPP mouse model. These data suggest that bitopertin may be effective in both treating the photosensitivity and modifying the hepatoxicity in EPP patients. A clinical trial in EPP patients has initiated to test this possibility.
Disclosures
Wu:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Xiang:DISC Medicine: Current Employment, Current holder of stock options in a privately-held company. Fleming:DISC medicine: Current holder of stock options in a privately-held company, Honoraria. MacDonald:Disc Medicine: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company. Schmidt:Disc Medicine: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal